-
Content count
3,057 -
Joined
-
Last visited
-
Days Won
5
Posts posted by MMI Enterprises
-
-
Rod,
This is not a one post thread and so claification about my belief on concentrations being key is not really needed. See here:
Not a chemist, so but the way I see it the main 'potential' to make problematic salts would be the starting concentrations of the metals (NaOH) and acid (which ever acid). In the course of cleaning or stripping we are already rinsing the main doses of metals with 5gpm+ before acidifying the wood.
The deeper point that perhaps could stand for some clarifying is that such graph/confirming info shows that if the concentrations were similar then the ability to go more acidic is hampered by the mandated buffering created. I used the info in a reverse deconstructing way. When I see it play out or proven that we are hampered is the point where I would say we dd not rid the deck of alkaline/metal. Your expounding is fine and all as we are all open but it is a tad different than what I expound so I wish to make that clear.
I say the acid does indeed find it's way in by nature of water being the common solvent. Hence the color flip. I am also saying I personally prefure to go more acidic and so is reason I keep the concentration higher than what the diluted/rinsed stripper concentration should need..
That said, Don't get me wrong folks, I do believe that a fair amount of alkalinity can not be rinsed entirely from the wood. Porosity or the fibrous lignin alike surely absorb and take away some the concentration from the main dose of chem that flows away while either applying or rinsing. I think it safe to say that what we start with in stripper concentration is not what we need to count on needing neutralized or overcome in order to head south towards acidic...no ability to go more acid equals a no to low salt/unbuffered situation. Just doesn't happen cause we rinse stripper sufficiently to prevent it.
-
What no gift buying yet Ron?..well be careful and beware of the dog house. It applies to b-days, anniversaries, as well as Xmas. Believe me I been expert at bad gifts or just plain missing dates altogether. I am getting better though with a little training.
-
I've actually learned to downstream using just that ballvalve that Russ is showing - I don't use the wand for much anymore.Elaborate please.. :)
-
Sorry..yer chat don't work Ron as the username imput field is too short to allow for my username. Doesn't accept a fake one neither..
-
Hey Beth can you check his IP address and see if it matches with any other screen names here and share with Ron M?Louis probably doesn't yet know of that other great site propowerwash.com (Pressure Wash Institute) or he would have started thread over there. It is like one of the top boards afterall..
-
Think they read the thing it's final rights..but your welcome to go ahead and resurrect the thing by trying out a DSer on the end of yer line. Some old coot said it worked but couldn't offer anything of what draw ratio was or if he drilled it or such.
-
That said there are those that don't use pressure washers when they clean many'a things eh folks?..just the same though Louis I think some are out for blood and want to catch you as really not knowing anything or string ya up for unintentional implying.... lol.. :)
-
I see.. thanx....Probably should always make clear of dif between cosco and costco the store.
-
Thanx for posting that site Ken...there's parts in it that speak to salts basically equalling buffers hence confirming my point.
Rick, I guess it would be interesting enough experiment but I don't think it will work to its fullest ability of making salt unless you do titration. The concentrations will need to be different depending for sure on what acid or base is used in order to end at their equivelance point. Besides their obvious starting ph's there are likely other factors I know nothing much of figuring such as starting water ph or temp, how old the chems , etc.. So but the use of 'equivelance point' is not a equal parts/measuring thing nor does it mean a neutral ph. As my link points out the 'end point' where the colored titration indicator additive shows up may not be exactly the equivelance point in all case neither. Refer to that page link or maybe it's sublinks on the indicators..maybe there is info ya can use if doing with citric acid.
Other prehaps more valueable experiments I suggest we take on would be to do some titrating with various branded stuff like citralic or hd80 along with their counterparts in raw form. Between that and generic ph strips we could confirm if label recommendations are worth a darn or if we can save some money or not.
In the end it does all depend what ph you want to end at in order to accommodate your stain or how bright ya want to go so that's why I mention the strips. Such won't work right with bleach as you proably know but probably fine with our other main wood chems.
-
Jon what is that ya sayin.... is that a Cosco ladder from Sams Club? Thought that was mainly a carseat brand. Out here we have warehouse stores called CosTco so just want to clarify who makes the ladders.
-
Louis these here folk don't use jugs..they use tanks! :)
-
I don't know...I thought post #18 was plenty apologetic and provided enough explaination for what he posted in this forum.
-
Terry,
The reports/survey we were talking about over here yesterday were about how they surveyed the teens about ethics with questions if they've shop lifted in last how many days or cheated on tests, etc. and the numbers were incredible. So incredible that a bunch of us discussing didn't believe the survey. I thought it sponsored by somebody with an agenda.
Do you think that was same thing?
-
imagine even the pilots anguish... that dude is gonna go through life always second guessing if he could have done something different the last few seconds. I'd probably end my career and be depressed as all get out...probably turn to booze at the very least.
-
Maybe they just real hot and those are all incoming texts?
..so that brings to mind the old net analogy.. This thread is useless without pics!! :)
-
link don't work.. that about the plane crash in San Delamo? Is horrible if that what your refering to.. poor guy lost his wife two daughter and motherinlaw... pure tragedy it is. If anything could ever make me just end it all it would be that. ;(
-
Matt, btw another thought on yer sanding issue...
You know besides the stain makeup being suspect of causing the tannin reaction you could also look to what you sanded with as possable cause of the darkening. Metalic varieties of paper can impregnate wood fibers with enough metal to where tannins do their thing once moisture arrives. Is just like the tea bagging/ebonizing wood process or any tannin type staining really.
When I use nylon pads on the big floor machine I have no chance of such problem.
-
I hear ya Dan...
that there is anything left to 'neutralize'.Just as easy this statement can mean there nothing left of base to take the acid to neutral.
One reason I suggest staying high enough on acid mix is that if yer way weak and you do have some base still present than yer bound to end up making some salt...same fer only lightly misting a deck wth pumpup..
If yer gonna do such a mist then perhaps it is best after a main flush of acid and mainly designed to get rid of persistant dark areas or tannin moving around..
-
Yup that's pretty much the English of what I sayin Rick.
Other than that..RS has been fine with yer wood so go with it.
Even though the rs site itself has some info or reasoning of their redwood only version I do know the aspects of the wood west versus the east of the big Miss can rightly be downplayed compared to either excessive extractives found in old time heartwood or redwood being rone in general. Heck it could be just that any fresher redwood no matter where from could respond badly to some standard formula. I heard the redwood not all much different these day whereever it comes and yet I've seen it go black. Just my experience with it is all on new boards which should be directly comparabel to what Matt said of his sanded wood.
-
yup..that what I do..rinse rinse rinse, especially during strip and yup even sometimes during brightening. :)
-
You've done the right thing Louis.. and so yer ok in my book.
Remember guys this is about the point when things either go really bad or really well far as people getting along... Enjoy the day is what I say!!
-
Too much exageration going on in my opinion about salt. Feel free to let me know what ya think...
Not a chemist, so but the way I see it the main 'potential' to make problematic salts would be the starting concentrations of the metals (NaOH) and acid (which ever acid). In the course of cleaning or stripping we are already rinsing the main doses of metals with 5gpm+ before acidifying the wood. I say acidifying as the normally used word in neutralizing is usually incorrect by way of the very nature of being able to go lower than would be expected from an equivelance/balance of both the acid and base involved. In the case of decent flushing of wood with oxalic (ethanedioic) acid you bypass neutral and haven't much chance of forming it's ions into salts. You aren't going to be making a buffered solution. If overwhelming amounts of salt had been formed said salts would buffer the overall ph solution preventing further acidifying. But generally it doesn't happen and I've never seen it on a deck and don't expect to neither. Note in Russell's thread there can be found confirmation that the process involves the acid going neutral. If you try to do a titration you would have to add back some alkaline cleaner to the acid to reach equivelance point.
..take a petri dish in a lab situation and place a sponge in it. Now dose the heck out of it with a titration indicator mix of hydroxide and phenolphthalien until full. It will be a redish pink color. Run it under faucet for hardly any time similar to rinsing stripper on a deck and it goes clear. Now flood dish/sponge with a oxalic solution. It won't go red.
On the acidic redwoods I deal with the effect is even that much worse in trying to make something of salt when you get down decently into fresher wood by way of cleaning or sanding.
Realize further that on yer darkened wood or yellowed wood you are pretty much looking at end effect rather than current situational amount of chems within. Confirm this by rinsing the wood for hours on end with neutral water..it'll never turn color until you brighten. btw same can happen on carpet and solution is something called brownout if I remember correctly. Example of the flip in color or brightness of wood is not really akin to titrating in my mind.
All that said yes it indeed possable that at some point any of us may leave some alkaline chems in wood by way of insufficient rinse and set ourself up for problems with a stain reacting badly or some salts being formed that don't flow away. I mean we are after all by nature of the equipment driving things into the wood and then relying somewhat on things mixing or absorbing by way of water solvency or Ken's mention of surfactance. Hey 2 stepping probably makes some salt eh?...
Here's some ref that points out speed of ph lowering/buffering and perhaps is something of confirmation of some the points I make:
"Titration curves for weak acid v strong base
We'll take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base.

Running acid into the alkali
For the first part of the graph, you have an excess of sodium hydroxide. The curve will be exactly the same as when you add hydrochloric acid to sodium hydroxide. Once the acid is in excess, there will be a difference.
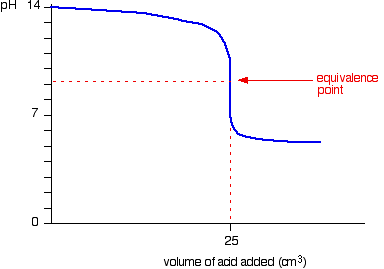
Past the equivalence point you have a buffer solution containing sodium ethanoate and ethanoic acid. This resists any large fall in pH."
edit: correction though- above acid is acetic rather than oxalic..spelling is similar so no they not specifically talking of our oxalic example but just the same ya probably can still see the points and it suitable example.
-
Why can't I do both?Sorry...I meant to say threw together with errors or outdated info.
So 'why can't' is cause ya can't do one while doing the other.
Now if ya can throw together site with solid info that works towards bettering the field then great and I say CAN.. :)
I respect your candid response in stating how that site came about.
As consolation I can say after reading every page you had up that I found plenty of solid stuff along with the bad that is worth keeping. Pretty good writing if ya ask me... If you were to go back and change somethings and offer up more facts of why some may or may not use this or that chem or choose this or that equipment, etc. then it could be good work. Regardless though of what ya end up with there will always be those that take issue if their way is not deemed the right way. Others may take issue if what is being spoken has room for personal choice yet those choices go unmentioned. Favoring one process or one equipment, etc. without mention of another rubs people wrong way I suppose in that it just doesn't point to homework having been done. You know what they say about pleasing all of the people all of the time..just can't be done. Much in cleaning is subjective.
To head things in right direction I'll address the muriatic as not all the places it was brung up contained disclaimer info of what it should generally not be used on and even then when it's use was cautioned there was not much info presented as to why.
1. muriatic is not metal inhibited so it eats metal found in many machines (that said we do have application equipment suitable)
2. muriatic eats/etches cement which can expose aggregate and can also quiken failure of reinforcing metal within by way of damaging it's protective oxidation through it's ph lowering.
..Both aspects can lead to dangerous disaster at grand scale if not respected.
When it is used on concrete its use is part of a more indepth restoration procedure involving prep for certain coating applications. Such involves some understanding/agreement of material loss.
-
granted..now if we could flush the potty posts we could get back on job.:)



Sanding before or after acid?
in Wood Cleaning & Restoration - Decks, Fences, etc.
Posted · Report reply
No appologies needed Rod..
On yer last statement about cellulose..Instead of defining what specific parts of the mass we call wood that can take on a ph or change in ph suffice it that I care not to guess. Perhaps we could suffice it to say that much of the structure would have to be able to be changed in order that we experience an alkaline or acidic situation. Our goal is changing it so it must be possable right? ..:)
The architecture, structure, or what have you is ordered in a fibrous way that can effect penetration of this or that and since lignin can be thought of as both the glue if you will or in some case as a seal that prevents things entering the rest of the ordered mass we have sorta no choice but to realize that it can be compromised, changed, or disolved (i.e-making pulp)....
As a mass, the terms fibrous lignin or fibrous cellulose werks well enough fer me...Whatever case, the ordered structure and/or absorbtion, etc. makes for whatever amounts of chem or ph we could claim the wood has left or displays...Rod do you by chance have any info on how the makeup of wood can mechanically perform concentrating duties to chems such as that of a filter? In case of a filter such takes place by way of the solvent travelling on through forcing a mechanical trapping. If the makeup of the mass is anywhere near as efficient as say compared to that of a reverse osmosis membrane then perhaps not much of what we term as our active chems can make it deeper then the next one. Weird, if not contradictory thought eh? Water might go bu not something else..haha. Maybe it does that on some micro cellular level but apparently, once again, doesn't show as such when brightening or flipping the color of an alkaline effected wood. Regardless, however strong the chemical concentrating could get by way of the solvent/water settling, pressure, absorbtion, etc. I would tend to think it can be still had at and diluted by the carrier/solvent that took it there in first place which is water. Hence rinse rinse rinse in case of at least the alkaline cleaners..
Hey besides all we have discussed I suppose we could discuss damage possable from leftover acids or alkalines. I imagine to tread there we would have to know something of a woods percentage of soluability or insoluability by specific acid or alkaline...anyways, I am rambling now..lol.
First paragraph here http://www.ipst.gatech.edu/faculty_new/faculty_bios/ragauskas/technical_reviews/Basics%20of%20Kraft%20Pulping.pdf explains something of lignin to where we can picture what it is and its duties.